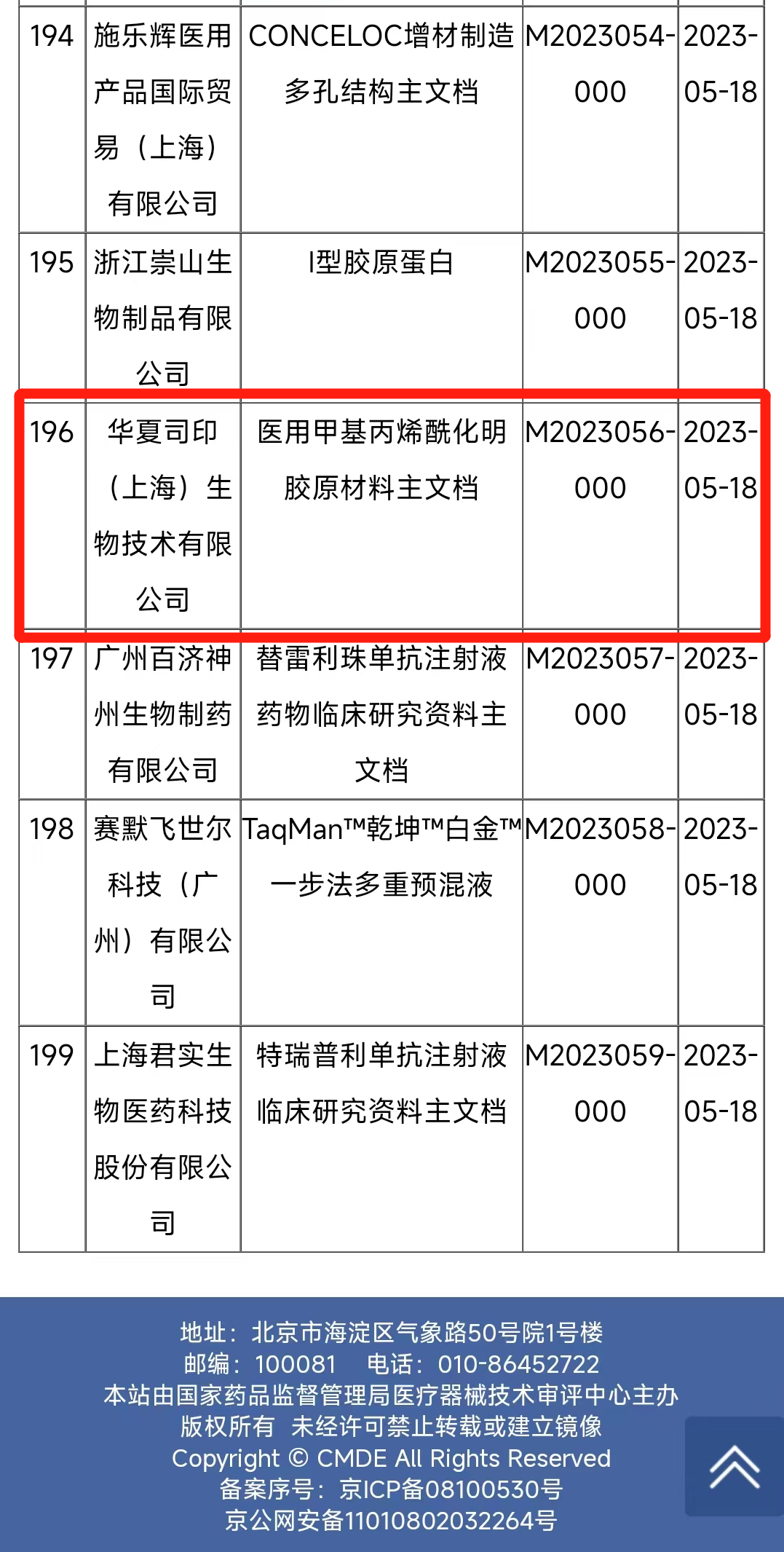
The picture shows the registration information publicity of the main document of the Medical Device Technical Evaluation Center (CMDE) of the State Drug Administration
In 2021, the State Food and Drug Administration issued the Announcement of the State Food and Drug Administration on the Registration Items of Medical Device Master Document, formally establishing the medical device master document registration system. When a medical device manufacturer is applying for medical device registration, the master document owner will issue a letter of authorization to the applicant for registration. When the regulatory body evaluates the application materials of medical devices, it may refer to the registered master documents according to the authorization letter for evaluation.
The medical grade GelMA raw material registered by SinoBioPrint conforms to the relevant national quality standards such as YY/T 1453-2016 "Method for the Characterization of Collagen I of Tissue Engineering Medical Devices" and "Pharmacopoeia of the People's Republic of China" (2020 edition). The methyl acrylyl substitution degree can be customized according to customer requirements (40%, 60%, 80% and 100% optional), the residual methacrylic acid (MAA) can be controlled below 100µg /g, endotoxin can be controlled below 0.5EU /mg, belonging to the high quality medical grade methyl acrylyl acylated gelatin raw material.
GelMA has been used by researchers as a foundation supporting material, widely used in the repair and regeneration of bone, cartilage, skin, tendon, blood vessel, cornea and so on. Based on GelMA, SinoBioPrint conducts clinical projects such as cartilage regeneration hydrogels, osteo-cartilage 3D-printed scaffolds and hip cartilage lining grafts. The registration of the "medical grade GelMA" raw material master document lays a solid foundation for the transformation of SinoBioPrint clinical project, and also indicates that this product can be used as one of the standard raw materials for product design and development of other domestic medical device manufacturers, which greatly saves the development time of device products and boosts the development of biological 3D printing and regenerative medicine.




